|
E-beam
Sterilization : A Unique Opportunity For Indian Medical
Device Industry

V C Petwal
Radiation Processing In-charge, ARPF & Head,
Radiation Processing Lab, Department of Atomic
Energy, Govt. of India
Raja Ramanna Centre for Advanced Technology,
Indore, Madhya Pradesh - 452 012
vikash@rrcat.gov.in |
Mr V C
Petwal , Head , Radiation Processing Lab, Raja
Ramanna Centre For Advanced Technology (RRCAT
),Department Of Atomic Energy , Government Of
India located in Indore. RRCAT is working in
non-nuclear frontline research in the area of
accelerator and laser technologies. The facility
is designed, developed, commissioned and operated
by RRCAT under the “AtmaNirbhar Bharat Abhiyan” of
government India and providing radiation services
for medical devices sterilization ( e-beam
sterilization ) . The facility is licensed by
Atomic Energy Regulatory Board (AERB), Food and
Drugs Administration (FDA) MP state and has ISO
9001:2015 and ISO 13485:2016 certification for
providing electron beam processing service for
sterilization of medical devices as per the
requirements of ISO 11137.
In a
free-wheeling interview to “Medical Plastics Data
Service", apart from introducing the first e-beam
sterilization facility in the country , the
process of sterilization, Medical Devices that can
be sterilized by this technique, its comparative
advantages as well as how the Indian Medical
Device Industry can take benefit from this
facility.
Raja
Ramanna Centre For Advanced Technology (RRCAT) has
set-up an electron beam sterilization facility at
Indore. Can you please briefly share over view on
this facility? |
Raja Ramanna Centre
for Advanced Technology (RRCAT) is a Premier R&D
Institute of Department of Atomic Energy, Government
of India. RRCAT is working in non-nuclear frontline
research in the area of accelerator and laser
technologies. RRCAT has setup an in-house developed 10
MeV electron linear accelerator (Linac) technology
based radiation processing facility at Indore, Madhya
Pradesh. The facility is designed, developed,
commissioned and operated by RRCAT under the
“AtmaNirbhar Bharat Abhiyan” of government India and
providing radiation services for medical devices
sterilization. The facility is licensed by Atomic
Energy Regulatory Board (AERB), Food and Drugs
Administration (FDA) MP state and has ISO 9001:2015
and ISO 13485:2016 certification for providing
electron beam processing service for sterilization of
medical devices as per the requirements of ISO 11137.
With these credential and approvals, this is the first
e-beam facility in the country providing commercial
radiation sterilization service to the regulated
medical devices.
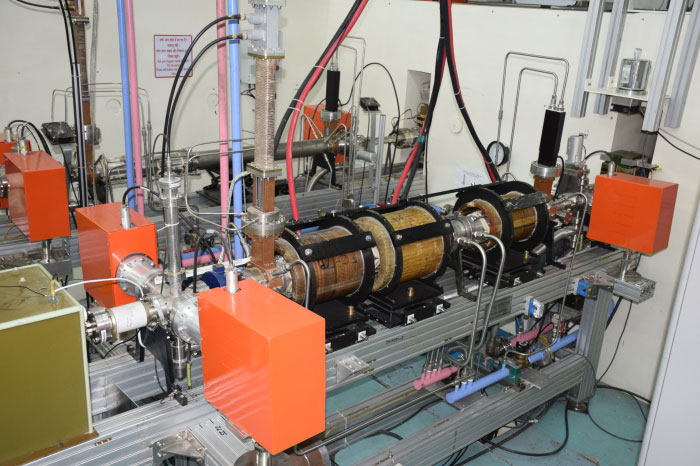
RRCAT Linac based
e-beam sterilization facility at Indore
Different medical devices
sterilized with e-beam
Apart from commercial
sterilization of medical devices, the e-beam facility
is being used by many institutes, universities and
industries across the country for variety of research
applications such as sterilization of pharmaceutical
API, irradiation of seeds for developing new crop
varieties, modification of semiconductor device
properties, colour modification of gem stones etc.
We understand you have already started offering this
facility to the Industry. Can you please explain how
is this facility being offered to Indian Medical
Device Industry?
Yes,
Being the first e-beam
facility in the country, various protocols/ process/
procedures are designed & developed to fulfil the
requirement of Schedule IV & V of MDR-2017 and ISO
13485. The facility is critically audited by AERB,
FDA, CDSCO notified bodies and after taking regulatory
approvals & ISO certifications, RRCAT started offering
radiation sterilization services to Risk Class-A and
Risk Class-B medical devices on commercial basis.
As the Government of
India is celebrating the “Azadi ka Amrit Mahotsav”, to
commemorate 75 years of progressive India and it’s
achievements, DAE has decided to celebrate it by
providing the e-beam sterilization service with
indigenous Linac technology, at concessional rates.
Medical device manufacturers from all around the
country are welcome to take the advantage of the novel
e-beam technology to sterilize their devices. Detail
information on the procedure to be followed for
availing sterilization service is provided on the
link:
https://www.rrcat.gov.in/organization/cat/incubation/ebrpf.html.
In short, the facility
have all the technical capabilities and control on
process to provide e-beam sterilization services to
Medical Device Industry, in conformance with
international standard.
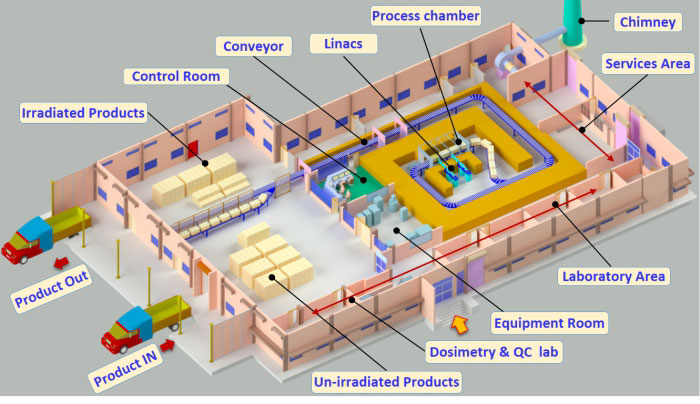
Layout of electron beam
radiation processing facility
Currently the Indian Industry is using either ETO
Sterilization or Gamma Ray Sterilization process. Can
please explain advantages of using e-beam
sterilization.
Sterilization by EtO
requires long exposure time (typically 5-6 days),
leaves chemical residue on devices and requires
expensive breathable packaging for medical devices.
Ionizing radiation is an attractive method for
terminal sterilization of medical devices. Electron
beam sterilization is equivalent to sterilization
performed with Co-60 Gamma radiation. For example
Gamma irradiation, e-beam doesn’t leave any residue
and requires no post processing quarantine time. The
processing time in electron beam is faster and based
on ON/OFF technology. Electron beam is eco-friendly,
and inherently safe and secure technology, due to
which its application is increasing at a rapid rate.
The medical devices are sterilized in their final
packaging using the penetrating power of electron
beams (without the need for opening packing).
Are there any limitation of using e-beam sterilization
particularly with respect to penetration or any other
output?
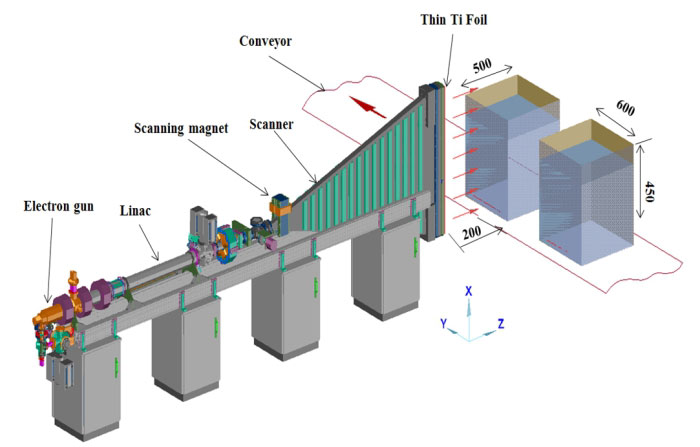
Typical scenario of
radiation processing using electron beam
|
