|
Polymer Ceramic
Hybrid Acetabular Liner: Bench to Bedside Translation
|
 |
Dr. Bikramjit Basu
Professor at the Materials Research Center IISc,
Bangalore |
According to a published report of the
World Health Organization (WHO), about 190 million
adults suffer from osteoarthritis and related
disabilities worldwide. This is commensurate with the
fact that the number of revision surgeries has
increased at about the same rate as the number of
primary surgeries of joint replacement due to
prostheses failures. Currently, there are more than
100,000 cases every year of Total Hip Arthroplasty (THA)
procedures in India. The available articulating
joint-implants generally offer a trouble free life for
about 10-15 years, which is inadequate, considering
the increased lifespan for humans in many developing
nations. Therefore, a search for ideal prosthetic
materials together with treatment methods,
reconstructive solutions and surface designs is
currently being pursued in the field of orthopedic
biomaterials.
The global orthopedic market was
approximately 30% of the total implants market and the
market shares of segments in 2020. The growth rate of
the need for orthopedic implants is estimated to be
more than 25% per annum for the next five to six
years. The global hip replacement implant market is
expected to grow at a Compound Annual Growth Rate (CAGR)
of 3.0% during the forecast period 2017–2023 to an
aggregate of $7,150.0 million by 2023. The overall
trend therefore shows a steady, yet slow expected
market growth in the THR application domain. The THR
market is further sub-divided into three sectors:
total hip replacement, partial hip replacement and
revision and hip resurfacing. Although several hip
implant options are available commercially, these are
currently imported and there are no affordable
indigenous alternatives.
In a decade-long research program,
Prof. Bikramjit Basu’s research group at Indian
Institute of Science, Bangalore, made
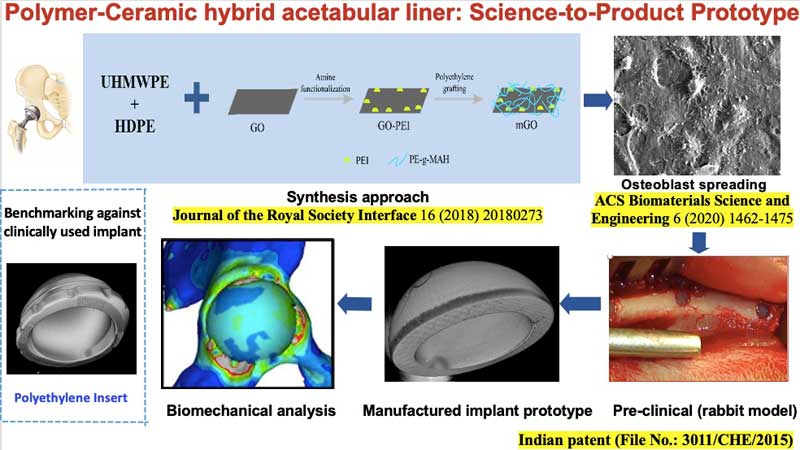
important breakthroughs in developing
three different generations of polymer-ceramic hybrid
acetabular sockets, namely HDPE-HA-Al2O3 hybrid
composites, polyethylene grafted graphene oxide (GO)
reinforced high density polyethylene (HDPE) composites
and lately, a UHMWPE-HDPE blend with surface modified
GO reinforcement. For example, a new synthesis
approach was developed to chemically couple GO in
polymer blends, which resulted in high mechanical
strength (~65 MPa) and wear resistance properties with
acceptable biocompatibility. Research on this
technology has also been conducted at IIT Kanpur.
Gamma irradiation of the acetabular
liner was shown to help in improving wear resistance,
hardness and coefficient of friction, without
compromising on biocompatibility. Improved wettability
and surface polarity after gamma-ray sterilization
further supported cell adhesion and stability of
coefficient of friction. The resulting wear debris
were found to be non-toxic. Optimum implant design was
determined using Finite Element Analysis and
theoretical wear analysis, and was customized
according to the bone condition and body weight of the
patient. It was shown that implant stiffness could be
optimized to be close to the natural strain profile
within bone.
The scalability for manufacturing of
acetabular liners (44, 46 or 48 mm outer diameter with
8 mm wall thickness) with acceptable surface finish
has been established at the prototype level. The new
implant design was accomplished by biomechanical
analysis of principal stresses in periprosthetic bone
around the acetabular joint, and current efforts are
underway to adopt machine learning algorithms to
accelerate the implant design. The augmented bone
tissue regeneration around the variants of hybrid
composite were demonstrated in both cylindrical and
segmental defect models in femurs of experimental
rabbits for a period of up to 26 weeks. |
|
|
|
|
Advertisers'
Index
|
|
Accuprec Research Labs Pvt. Ltd., India |
|
Ambica Medicare Engineering, India |
|
Nu-Vu Conair Pvt. Ltd., India |
|
Celanese Corporation, India |
|
CLS Pvt.
Ltd., India |
|
Carclo Technical Plastics Pvt. Ltd.,
India |
|
Covstro India |
|
ET Elastomer Technik, Germany |
|
Eewa Engineering Co. Pvt. Ltd., India |
|
Ineos Styrolution India Ltd., India |
|
I-Kare Polyalloys Pvt. Ltd., India |
|
KLJ
Group, India |
|
Lubrizol Advanced Materials India Pvt.
Ltd. |
|
Lyondellbasell, India |
|
Mediscient Devices (OPC) Pvt. Ltd., India |
|
Milliken & Company, India |
|
Maider Medical Industry Equipment Ltd.China |
|
Mega Compound Co. Ltd., China |
|
Milacron India Pvt. Ltd., India |
|
GLR Laboratories
Pvt. Ltd., India |
|
HighRichja Precision Extrusion Machinery Co. Ltd., China |
|
PVC Colouring Compounding & Processing,
India |
|
Qosina,
USA |
|
Raumedic AG |
|
San
Printech Pvt. Ltd., India |
|
Schottli, Switzerland |
|
SMC Medical Manufacturing Pvt. Ltd.,
India |
|
Steri Techno Fab, India |
|
Tekni-Plex India Pvt. Ltd., India |
|
Twist Engineering Works,India |
|
Airways Surgical Pvt. Ltd., India |
|
Alpha Medicare and Devices Ltd., India |
|
Alpha Therapeutics Pvt. Ltd., India |
|
Angiplast Pvt. Ltd., India |
|
Beacon Plastics, India |
|
Delux Surgical, Inida |
|
Jain Rubbers Pvt. Ltd., India |
|
New
Vimko Plastics, India |
|
Operon Strategist, India |
|
R.R. Patel Gases (P) Ltd., India |
|
SEC Global Consulting & Initiative LLP,
India |
|
Surgi Pack India Pvt. Ltd. |
|
Vinit Performance Polymers Pvt. Ltd., India |
|
Amigo Surgi Care
Pvt. Ltd., India |
|
Apex Medical Devices, India |
|
Jimit Medico Surgicals Pvt. Ltd. |
|
Life-O-Line Technologist, India |
|
Mesco Surgical, India |
|
Morrisons
Lifecare Pvt. Ltd., India |
|
National Healthcare, India |
|
Pharmadocx, Inida |
|
S. Nath & Co., India |
|
Unikal Consultants, India |
|
