|
Medical Device & In
Vitro Diagnostic Devices:
MDR 2017 and Industry Preparedness
|

Dr. Sanjeev Kumar Gupta
Managing Consultant,
Intrust-Consulting LLP |
Since 1989 the regulation of
Medical Device (MD) and IVD Industry in India has
been growing consistently under Drugs & Cosmetics
Act 1940. Several notifications were issued in 1989,
2002 and 2005 covering 16 categories of MD-IVD with
in the purview of this Act. Several awareness
programs were organized by The All-India Drugs
Control Officers Confederation (AIDCOC) and Indian
Pharmaceutical Graduate Association (IPGA) for
different stake holders. India’s regulatory body
CDSCO consisting of Central Licensing and State
Licensing Authorities further built its capacity in
regulatory matters of MD-IVD under a WHO Capacity
Building Program at NIPER, Mohali.
|
Subsequently, close interactions between regulatory body
and industry associations (AiMED, ADMI) lead to
development & notification of Medical Device Rules 2017
paving way for structured regulation of MD-IVD in
country. From 1st January, 2018 these Rules brought a
paradigm shift in regulatory procedure for MD-IVD in
country along with clarity on classification of devices,
requirement of technical documentation, customization of
application forms, fee structure, timelines. This helped
several manufacturers to prepare themselves and submit
applications on easy-to-use MD-Online Portal of CDSCO.
More so a manufacturer specific dedicated dash board
added value for each user. Large number of
manufacturer’s completed their transition of licenses
from D&CAct to MDR2017 by end of 2019 with many more in
progress. Simultaneously regulation was preparing to
bring more MDIVD under the ambit of MDR Rules 2017.
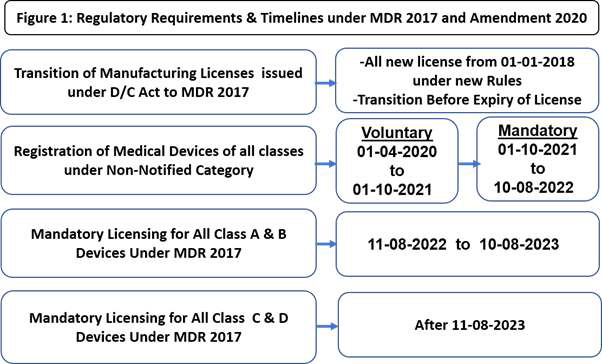
Two gazette notifications which were published in
February 2020 were significant. One expanded the
definition of medical devices (Refer Box 1) and another
advised the actions to be taken by industry (Figure 1).
Before it could get into industry awareness sessions the
country was hit by pandemic. As a blessing in disguise
entire country learnt about Ventilators, PPEs, VTM, RNA-
Extraction Kits, RTPCR, Antibody Tests and Antigen Tests
were all over NEWS papers and TV. Many also learnt about
ICMR and CDSCO for the first time as Import Permissions
were given and Emergency Manufacturing Licences were
given. MD-IVD-Regulation all were at forefront.
Box-1 Definition of Medical Devices under Medical
Device Rules 2017 and Amendment 2020
| Before 11th February
2020 “medical device” means,-
(A) substances used for in vitro diagnosis and
surgical dressings, surgical bandages, surgical
staples, surgical sutures, ligatures, blood and blood
component collection bag with or without anticoagulant
covered under sub-clause (I),
(B) substances including mechanical contraceptives
(condoms, intrauterine devices, tubal rings),
disinfectants and insecticides notified in the
Official Gazette under sub-clause (ii),
(C) devices notified from time to time under
sub-clause (iv), of clause (b) of section 3 of the
Act; Explanation: For the purpose of these rules,
substances used for in vitro diagnosis shall be
referred as in vitro diagnostic medical device. |
From 11th February 2020
All devices including an instrument, apparatus,
appliance, implant, material or other article, whether
used alone or in combination, including a software or
an accessory, intended by its manufacturer to be used
specially for human beings or animals which does not
achieve the primary intended action in or on human
body or animals by any pharmacological or
immunological or metabolic means, but which may assist
in its intended function by such means for one or more
of the specific purposes of ―
(I) diagnosis, prevention, monitoring, treatment or
alleviation of any disease or disorder;
(ii) (ii) diagnosis, monitoring, treatment,
alleviation or assistance for, any injury or
disability;
(iii) investigation, replacement or modification or
support of the anatomy or of a physiological process;
(iii) (iv) supporting or sustaining life;
(iv) (v) disinfection of medical devices; and
(v) (vi) control of conception. |
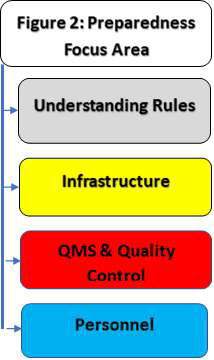 A
large number of manufacturers and importers of newly
notified medical devices have not seen and experienced
the requirements given in MDR 2017 owing to the fact
that these were not regulated until February 2020. The
Figure 2 shall help them know that compliance to these
Rules needs several levels of preparedness such as a)
clear understanding of Rules, b) requirements of
infrastructure, c) QMS & Quality and d ) requirements of
personnel. It is easy for some and nightmare for many.
The different chapters, schedules and appendix of MDR
2017 and Amendment 2020 offer excellent guidance to
prepare for all these segments. Industry can also take
services from Consultants who have medical device domain
knowledge and experience. It would be pertinent and
effective to undergo a comprehensive training for clear
understanding. All kinds of conflict of interests shall
be addressed while hiring a consultant and all
consultancy shall be availed against a signed quality
agreement as per firm’s outsourcing SOP. A
large number of manufacturers and importers of newly
notified medical devices have not seen and experienced
the requirements given in MDR 2017 owing to the fact
that these were not regulated until February 2020. The
Figure 2 shall help them know that compliance to these
Rules needs several levels of preparedness such as a)
clear understanding of Rules, b) requirements of
infrastructure, c) QMS & Quality and d ) requirements of
personnel. It is easy for some and nightmare for many.
The different chapters, schedules and appendix of MDR
2017 and Amendment 2020 offer excellent guidance to
prepare for all these segments. Industry can also take
services from Consultants who have medical device domain
knowledge and experience. It would be pertinent and
effective to undergo a comprehensive training for clear
understanding. All kinds of conflict of interests shall
be addressed while hiring a consultant and all
consultancy shall be availed against a signed quality
agreement as per firm’s outsourcing SOP.
The Good Manufacturing Practices (GMP) for MD-IVD have
been covered under Quality Management System (QMS)
explained under Fifth Schedule of MDR2017. While
licensing process application does not require ISO13485
certificate, the registration process requires ISO 13485
certificate to be uploaded on portal. This is
significant as this requirement necessitates
implementation of ISO13485 and a certification. All
manufacturers and importers shall make all efforts to
understand ISO13485 and integrate their existing
processes with processes required by standard. |
|
|
|
Advertisers' Index
|
|
Accuprec Research Labs Pvt. Ltd., India |
|
Ambica Medicare Engineering, India |
|
Nu-Vu Conair Pvt. Ltd., India |
|
Divya Steri Solutions Pvt. Ltd., India |
|
ET Elastomer Technik, Germany |
|
Eewa Engineering Co. Pvt. Ltd., India |
|
Ineos Styrolution India Ltd., India |
|
I-Kare Polyalloys Pvt. Ltd., India |
|
KLJ
Group, India |
|
Lubrizol Advanced Materials India Pvt.
Ltd. |
|
Kuraray India Pvt. Ltd., India |
|
Maider Medical Industry Equipment Ltd.China |
|
Medicall 2019, India |
|
Ferromatik Milacron India Pvt. Ltd., India |
|
GLR Laboratories
Pvt. Ltd., India |
|
Pashiba Lifescience, India |
|
Plastivision India |
|
Pradeep Surgipack, India |
|
PVC Colouring Compounding & Processing,
India |
|
Qosina,
USA |
|
Raumedic AG |
|
SMC Medical Manufacturing Pvt. Ltd.,
India |
|
Sterimed Medical Devices (P) Ltd., India |
|
Steri Techno Fab, India |
|
Tekni-Plex India Pvt. Ltd., India |
|
Twist Engineering Works,India |
|
Yuhuan Shengjiu Mould Co., Ltd.,
China |
|
Airways Surgical Pvt. Ltd., India |
|
Alpha Medicare and Devices Ltd., India |
|
Alpha Therapeutics Pvt. Ltd., India |
|
Ami
Polymer Pvt. Ltd., India |
|
Angiplast Pvt. Ltd., India |
|
Appasamy Associates, India |
|
Beacon Plastics, India |
|
Delux Surgical, Inida |
|
Ignisol Mediplas Corporation, India |
|
Jain Rubbers Pvt. Ltd., India |
|
Operon Strategist, India |
|
R.R. Patel Gases (P) Ltd., India |
|
Proven Trade Contacts, India
|
|
Sanidhya
Enterprise, India |
|
Surgi Pack India Pvt. Ltd. |
|
Unikal Consultants, India |
|
Vinit Performance Polymers Pvt. Ltd., India |
|
Aircity, India |
|
Amigo Surgi Care
Pvt. Ltd., India |
|
Angel Products, India |
|
Apex Medical Devices, India |
|
Jimit Medico Surgicals Pvt. Ltd. |
|
Kavya Packaging, India |
|
Life-O-Line Technologist, India |
|
Mesco Surgical, India |
|
Morrisons
Lifecare Pvt. Ltd., India |
|
National Healthcare, India |
|
Pharmadocx, Inida |
|
S. Nath & Co., India |
|
Unikal Consultants, India |
|
Venus
Industries,India, Mobile : 9825747495 |
|
Zinkal Products, India |
|
