|
Low Risk, Patient
Friendly Microneedle Arrays: An Emerging
Medical Device for Enhanced Local/Systemic, Transdermal
Drug Delivery
|

Dr. Manish Nivsarkar
Dept. of Pharmacology and Toxicology,
B.V. Patel, Pharmaceutical
Education and Research Centre,
Ahmedabad. |

Dr. Viral Shah
Dept. of Pharmaceutics,
B.V. Patel, Pharmaceutical
Education and Research Centre, Ahmedabad |
ABSTRACT :
There exist distinct pathways for drug delivery to body to
achieve maximum therapeutic effects. Transdermal drug
delivery system has been recognized as one of the
potential alternative to conventional injection methods
because, skin is easily accessible for drug
administration. The Stratum corneum acts as a major
barrier in systemic or topical delivery of the drug via
skin. Transdermal administration of drugs is feasible only
for low molecular weight and moderately lipophilic drugs.
The biomolecules-based bio-therapeutics market is
expanding with the development of genetic engineering and
proteomics. Delivery of biomolecules-based
bio-therapeutics by achieving controlled disruption of the
skin without losing protective function of the skin is the
current need. Microneedles (MNs) based transdermal drug
delivery system can enhance the skin permeability of
hydrophilic drug substances and bio-therapeutics by
temporarily rupturing the skin barrier layer physically.
Various types of MNs have been designed and evolved. The
present review article would focus on the application of
low risk, patient friendly microneedle arrays as an
emerging medical device for enhanced local /systemic,
transdermal drug delivery.
INTRODUCTION :
Background
The pathway for drugs
to achieve therapeutic effects comprises distinct phases
of delivery in the body. Currently, most
biopharmaceuticals are administered by parenteral route
using hypodermic needle based injection systems. However,
treatment by injection requires a visit to the hospital or
clinic for the administration. In addition, hypodermic
needles should be disposed of under specific protocols
because reuse can be another path for disease infection.
Also the injection based systems have not received patient
acceptability till date. To resolve these problems,
pharmaceutical companies are currently focused on the
design of biopharmaceutical delivery by non-conventional
routes such as transdermal drug delivery systems.1-5
Development of a transdermal drug delivery system has been
of interest as one of the alternatives to conventional
injection methods because skin is the most easily
accessible site for drug administration, transdermal
formulations are easy and convenient to use and they would
likely have better patient compliance than hypodermic
needles.
The role of the
integumentary system which is composed of the skin is the
regulation of interactions between the body and the
external environment for the purpose of protecting the
body. The protective function of the integumentary system
composed of skin resides in the outer layer of the skin,
the stratum corneum. Stratum corneum prevents entry of
noxious chemicals, dangerous microorganism and dehydration
of the body by controlling water loss. Thus Stratum
corneum acts as a major barrier in systemic or topical
delivery of the drug via skin. It is observed that
transdermal administration of drugs is feasible only for
low molecular weight and moderately lipophilic drugs. It
is difficult to deliver high molecular weight and
hydrophilic biomolecules drugs into the body without using
hypodermic needles.
The barrier
properties of skin
Transdermal drug delivery systems have
been investigated for a long time due to the advantages
described above. However, the skin is exposed to external
environments and has become evolved into an efficient
barrier to prevent entry of harmful chemicals and
microorganisms. These barrier properties of the skin
result from the histological layers: the subcutaneous
tissue, the dermis layer, and the epidermis layer as shown
in Figure 1.
The skin consists of two main parts;
the outermost layer, epidermis, and the inner connective
tissue layer, dermis, as shown in Figure 1. The dermis
layer is 3-5 mm thick and the major component of skin. It
is composed of mostly collagen fibrous protein providing
the mechanical properties of skin. The vascular
structures, such as blood or lymphatic vessels, nerve
endings, and various glands, are in this layer to perform
nutrient/waste exchange, create sensation, and regulate
body temperature controlling, and
so forth. From the standpoint of transdermal drug
delivery, the vasculature elements in the dermis layer are
the absorption sink causing the concentration gradient of
drug diffusion from the exterior into skin and are the
driving force for drug permeation.
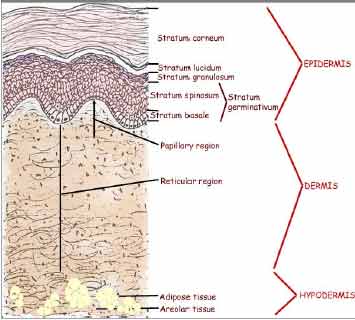
Figure 1 Schematic illustrations of
skin, stratum corneum and viable layer of skin
The epidermis layer
creates the barrier function against transdermal drug
delivery. It has complex multiple layers of cells in the
differentiation processes with the changes of structure
and the lipid content of skin. While cells in the lower
layers of epidermis, the viable epidermis, contain typical
organelles like mitochondria, the outermost epidermis
layer, the stratum corneum, is a horny layer with
approximately 10-15 μm thickness and composed of dead
cells, which are the final product of the differentiation
process. A typical horny cell has an amorphous structure,
created by dead keratinized cells with approximately 30-40
μm of diameter encompassed by multiple lipid bilayers.
This envelope
structure, in which keratinized cell and lipids are
continuously overlapped with each other. Because of this
‘brick and mortar‘ structure of stratum corneum , stratum
corneum is the key layer of the skin barrier for
regulating the flux of molecules from the inside to the
outside of the body and vice versa. Due to the stratum
corneum barrier, only small molecular weight and
moderately lipophilic drugs are able to diffuse through
the skin at a therapeutic rate. Consequently, various
transdermal methods have been designed for enhanced
transdermal delivery of hydrophilic or large molecular
weight drugs by modulating the barrier properties of
stratum corneum or disrupting it or breaking it or
removing.
Transdermal drug
delivery systems
Historically, a
primitive type of transdermal drug delivery patch was used
in the form of a medicated plaster several hundred years
ago. This prototype of a transdermal drug patch simply
contained herb extracts composed of small molecules such
as menthol and methyl salicylate used to soothe
inflammation in muscle or to help healing of bone
fractures by inducing a local analgesic effect.
While the barrier
properties of skin have been a subject of scientific
debate since the early 1900’s, it was shown that these
properties reside in stratum corneum and drugs can or
cannot permeate stratum corneum depending on their
water/oil partition coefficient around 1950’s. These
scientific findings triggered development of transdermal
drug delivery systems for systemic therapy. In early
1970’s, the first transdermal drug delivery system for 3
days of systemic effect of scopolamine against motion
sickness, Transderm-Scop® was developed by Alza and
approved for the USA market in 1979. Since then, various
types of transdermal drug patches have been designed for
systemic effect at controlled rates.
For patch types of drug
formulations, it has been suggested that transdermal
administration for systemic effects might be limited to
small and moderately lipophilic drugs because patches are
based on the passive diffusion of drugs from the reservoir
of a patch to skin. Thus, an active transdermal drug
delivery system has been sought to deliver hydrophilic or
large size drugs which are bio-therapeutics that cannot
permeate through intact stratum corneum. These active
delivery systems should bring an integrity change of
stratum corneum or the physical removal and breakage of
stratum corneum to increase the permeability of the skin.
To create an integrity
change in stratum corneum, chemical treatment of the skin
surface or the use of energy application was studied such
as chemical enhancement. with various chemicals,
sonophoresis with ultrasound, and Iontophoresis or
electroporation with electrical energy. For the removal or
breakage of stratum corneum, minimally invasive methods
were designed, such as the jet injector, microneedle and
skin ablation treatment.
However, the use of
chemical enhancers has been limited due to skin irritation
and the inability to deliver large size drugs.
Sonophoresis uses ultrasonic energy to increase the
permeation of drugs through skin. Iontophoresis uses an
electrical gradient for the transport of charged drugs.
Electroporation applies a relatively higher voltage (~100
V) pulse to skin than iontophoresis does, but the electric
field lasts for a shorter time, usually 10 μs –10 ms.
|
