|
Development of Plastics Injection Molded Medical Device: A
Systematic Approach is Key to Success
|

Mr.
Nikhil Patel
Director
Credence Management Corporation, Vadodra, Gujarat |
The Indian health care
industry is taking great strides. In the last few
decades, the industry has grown to the status of a
leading sector in the country with a sizable base.
India is doing extremely
good in plastic disposables and low end plastic
injection moulded medical devices. Unfortunately, 80%
of our high-end medical devices are imported from
other countries; on other hand there is 60% of export
of or indigenously produced medical devices,
especially disposables medical.
This is due to lack of
innovation, lack of systematic approach to the
development, Absence of good product design
capability, Limited Knowledge of Manufacturing and
Quality Assurance, limited world class manufacturing
facility to open market and unclear regulatory support.
There is clear need of
applying systematic approach to the device development,
what developed countries in US and Europe are following
from design brief to validations to mass manufacturing of
medical devices. |
Following structured
development practices will definitely change the scenario
of India- at present not being good at development of
high-end plastics medical devices.
Itís essential to take project
through each step to have world class quality device with
full functionality and long term reliability.
Medical-device development has
become increasingly complex in recent years. The advent of
new technology, stricter regulatory requirements, and the
ever increasing role of reimbursement for successful
commercialization require careful planning and strategy
setting, coordinated decisions, and consistent, rigorous
processes. The design and implementation of such processes
often captured in development models and related
standard-operating procedures have become key to
commercially successful devices.
 I
have tried putting together outline of structured
methodology for development of plastics injection moulded
medical devices. It is giving an idea of each development
stages like; Preparing Design Brief, Concept Development,
Product design, DFM, DFMEA, Design verification and
validation (DQ), Engineering Drawing, Proto tool
development, Production Tooling design and development,
Process development and validation (IQ,OQ,PQ), Product
testing and validation, product approvals etc. I
have tried putting together outline of structured
methodology for development of plastics injection moulded
medical devices. It is giving an idea of each development
stages like; Preparing Design Brief, Concept Development,
Product design, DFM, DFMEA, Design verification and
validation (DQ), Engineering Drawing, Proto tool
development, Production Tooling design and development,
Process development and validation (IQ,OQ,PQ), Product
testing and validation, product approvals etc.
Product Definition,
Conceptualisation & Design:
A development process starts
from gathering customer voice, which is been captured in
the document called Design brief that focus on exactly
what needs to be achieved before any work starts on the
development. Regulatory and salutatory requirements should
be defined at the start of device development project.
Once the product requirements
are defined, a review of the existing intellectual
property within a specific market or pathology is also
conducted, as well as an early stage technology risk
assessment. Patent search is vital to avoid duplication of
R&D work and hence prevent cost of duplication and assess
novelty and patentability of own developments with a view
of applying for a domestic or foreign intellectual
property right.
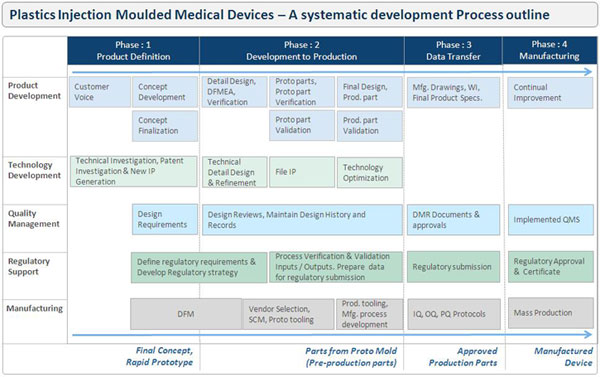
Multiple concepts are
generated for Functionality, Aesthetics and user
requirements. A cross-functional, project-core team is
selected, and a general project plan and timeline
developed. Design plan is been created where the team
leader is responsible for initiating and managing the
Design History File, a record indicating that the device
was developed as described in the approved design brief /
design plan.
Page
1 :
2 :
3 :
4 :
5 |
