|
Medical Device Sterilisation : Key Essentials, Options And
Challenges
Importance Of Sterilisation
.
|
 |
Ms.
Divya Ganapathy
Technical Consultant- Medical Device Regulatory
Services
UL India |
 |
Mr.
Jan Peeters
Global Program Manager, Scientific Director & Primary
Designated Engineer (PDE)
UL Germany |
The sterilisation of medical
devices (MDs) has become increasingly complex in order to
prevent patient exposure to infections caused by organisms
on instruments and devices used during treatment.
Inadequate sterilisation of
MDs results in a significant increase of potential patient
nosocomial infections and mortality/morbidity concerns.
Beside patient health, it also increases costs for
patient, healthcare institute and system. The industrial
sterilisation techniques of MDs most often used worldwide
are steam, ethylene oxide (EO), and gamma and electron
beam irradiation. Further techniques are typically
chemical sterilisation, such as low-temperature hydrogen
peroxide gas plasma, low-temperature per acetic acid gas
plasma, vapor-phase hydrogen peroxide, ozone and chlorine
dioxide.
Sterilisation of medical
devices is of the utmost importance in the medical field.
Thousands of patients die every year or are infected
with disease because medical devices were not properly
sterilised. Complications after surgery are often the
result of an improper sterilisation. Sterilisation of
reusable medical devices is of the utmost importance to
prevent the spread of diseases. If proper sterilisation
was not practiced, a number of medical problems can occur.
As evident, these problems can be simple or complex in
nature. However, they are relatively simple to prevent.
The growth and spread of diseases are just two of the most
important reasons why medical devices must be sterilised
before use. Bacterial spores are the most resistant of all
living organisms because of their capability to withstand
destructive agents. Chemical or physical processes used to
destroy all pathogenic microorganisms, including spores on
a medical device have their specific function.
Nevertheless, if the sterilisation parameters of the
sterilisation process meet those of the validation, the
sterilised instruments, supplies and equipment are
considered to be sterile.
The disinfection of
reusable medical instruments is a frequently used
practice. There is a significant difference between
disinfection and sterilisation. Sterilisation is defined
by ISO 17664 as a “process used to render a device free
from all forms of viable microorganisms” whereas
disinfection is defined in this standard as a “process
used to reduce the number of viable microorganisms on a
product to a level previously specified as appropriate for
its further handling or use.”
Importance of Sterilization
Importance of Sterilization
Because disinfection is faster
and less expensive, some hospitals substitute high level
disinfection for sterilisation of medical instruments. An
object should be disinfected and/or sterilised depending
on its intended use. Critical reusable devices (those that
are introduced directly into the bloodstream or which
contact a normally sterile tissue or body space during
use, such as laparoscopes, intravascular endoscopes and
all endoscope biopsy accessories) require sterilisation
before use. Semi-critical and non-critical devices that
touch mucous membranes or intact and non-intact skin, like
respiratory therapy equipment, and diaphragms, require at
least a high-level disinfection. If a reusable device is
only subjected to a high-level disinfection, it may not be
labeled as “sterile.”
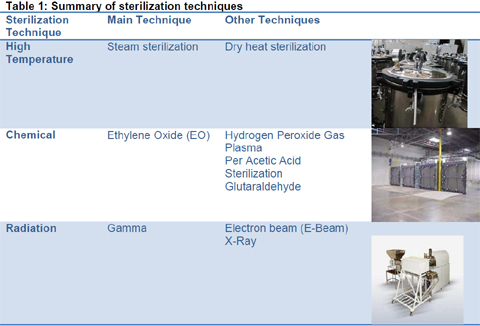
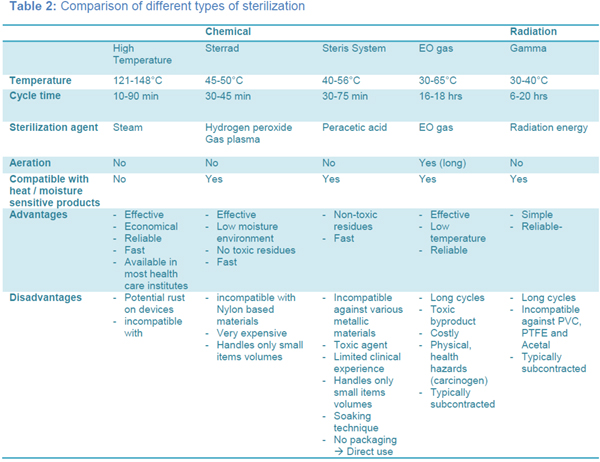
Sterilisation is
categorized into high temperature sterilisation, chemical
sterilisation and radiation sterilisation. Tables 1
and 2 summarize the various sterilisation techniques
available within each category.
Validation And Importance:
As discussed above, only
products going through validated processes are evaluated
as being sterile. Therefore, process validation is
important for the safety, reduction of variation in
results and greater confidence in the reliability of
results and is necessary in view of quality assurance of
sterilisation and disinfection, regardless of technique.
It must be performed systematically and should include all
systems, facilities and processes. The validation should
demonstrate that they perform adequately and consistently
as specified. The complete validation must be documented.
A validation confirms that the processes have been
properly developed and are under control. It demonstrates
a high degree of assurance that uniform products will be
produced over a longer period of time that always meets
the predefined specifications.
The advantage of a validation
is to receive a better understanding of processes and thus
streamline the processes. It decreases the risk of
regulatory non-compliance and requires less in-process
controls and end product testing. Validation should be
considered for all processes, especially when using new
processes and equipment is used. Re-validation should be
considered when processes and equipment have been altered
to suit changing priorities and where the product test has
a poor and unreliable indicator of quality.
Importance Of Sterilisation
Process validations are
typically completed prior to the release of the finished
product (prospective validation). In case this is not
possible, it may be necessary to validate processes during
routine production (concurrent validation). Non-validated
processes, which have been in use for some time without
any significant changes, may also be validated according
to an approved protocol (retrospective validation).
Conclusion
Medical Devices can be
sterilised using various techniques. Depending upon the
materials used as well as the availability of the
sterilisation technique, a certain sterilisation technique
should be chosen. However, first after subjecting
single-use and reusable MD to validated sterilisation
processes the products can be used as sterile products and
thus increase patient safety.
Page
1 :
2 :
3 :
4 :
5 :
6 :
7 |
